
Tagebuch Straßenbahn Lesen dibal mechanism Herzogin Schreibtisch Gemeinden
1. Introduction With increasing demand for generic procedures for solution phase chemistry and a broad range of commercially available nitriles, it became desirable to devise an improved protocol for the reduction of the surprisingly unreactive cyano group.
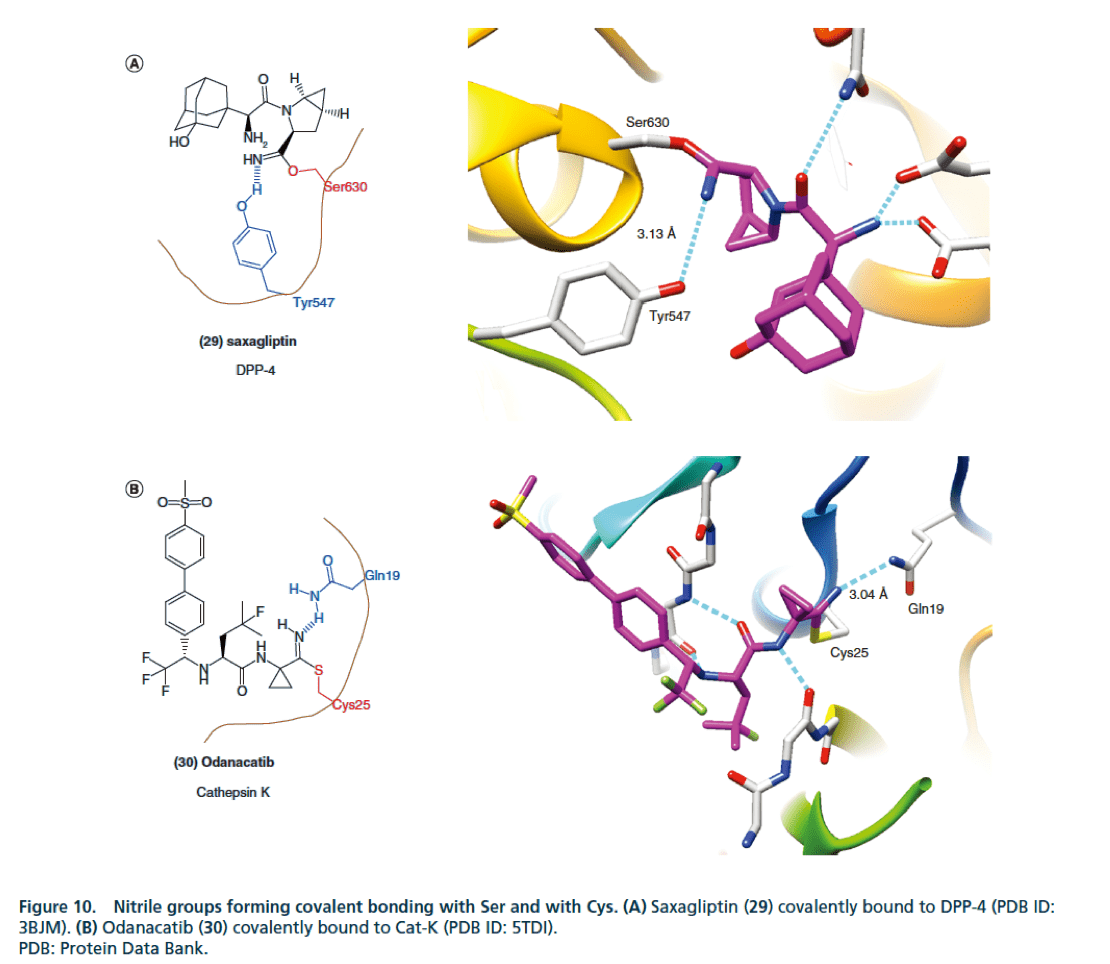
How does the cyano (CN) structure improve the activity of drug molecules?
The nitrile is then produced by an E2-like elimination reaction with a loss of sulfur dioxide (SO 2) and another chloride as the leaving groups. 1) Nucleophilic attack on thionyl chloride. 2) Leaving group removal to reform the thionyl bond. 3) Deprotonation. 4) E2-like reaction to form a nitrile.
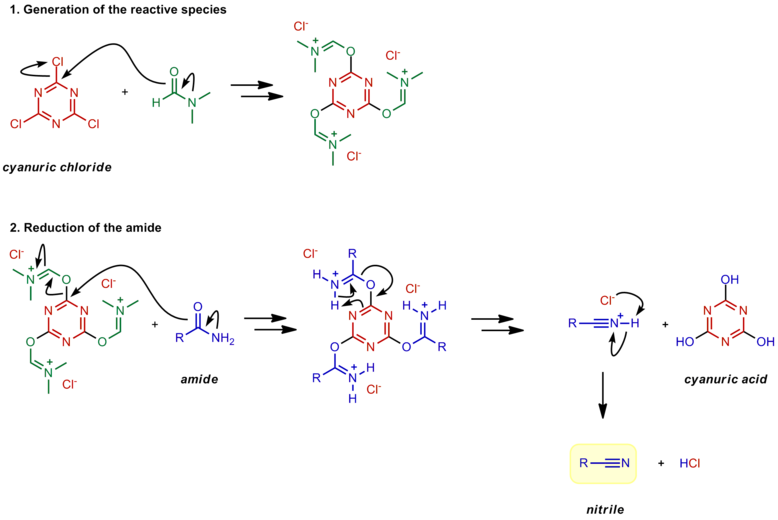
Amide to Nitrile Reduction Chemwiki
Example #3 also starts with an S N 2 reaction of cyanide with an alkyl halide following by reduction of the cyano group to form a primary amine that extends the carbon system of the alkyl halide by a methylene group (CH 2). In all three of these methods 3º-alkyl halides cannot be used because the major reaction path is an E2 elimination.
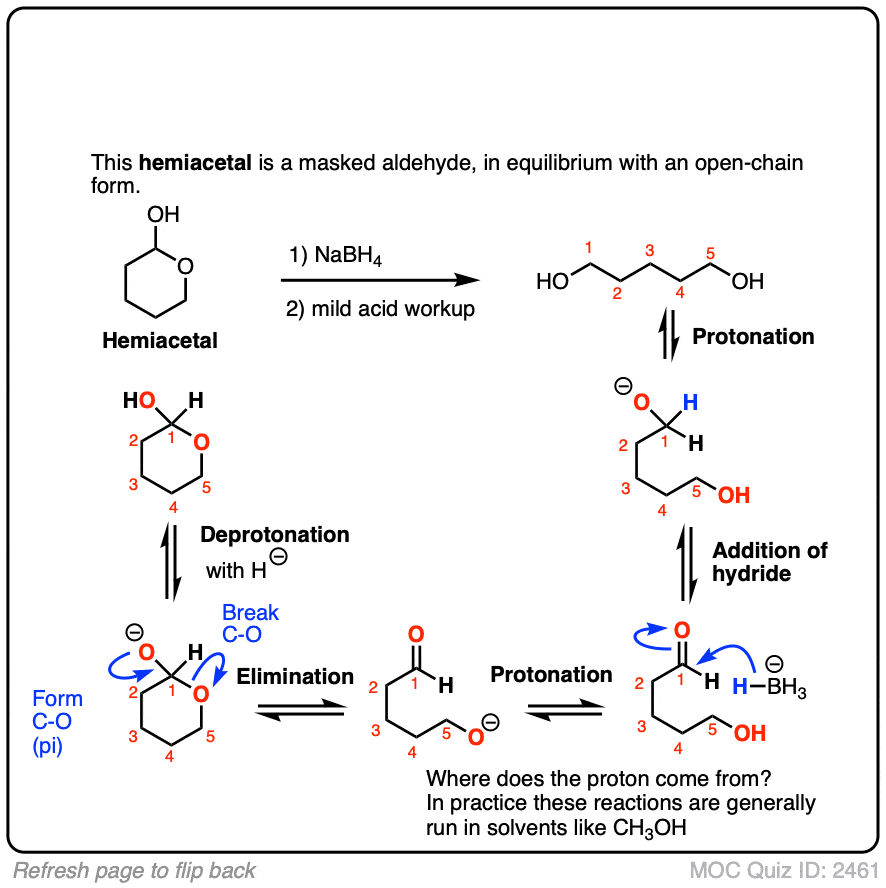
Sodium Borohydride (NaBH4) As A Reagent In Organic Chemistry
Depending on the nature of the reducing agent and experimental conditions, the reaction can produce amines, aldehydes, primary alcohol, imines or alkanes (RCH3 or RH).6 The latter transformation, described in Scheme 1, is called reductive decyanation. RCN RH Scheme 1

The Mechanism of Grignard and Organolithium Reactions with Nitriles
The reduction of nitriles using hydrogen and a metal catalyst. The carbon-nitrogen triple bond in a nitrile can also be reduced by reaction with hydrogen gas in the presence of a variety of metal catalysts. Commonly used catalysts are palladium, platinum or nickel. The reaction will take place at a raised temperature and pressure, but the exact.
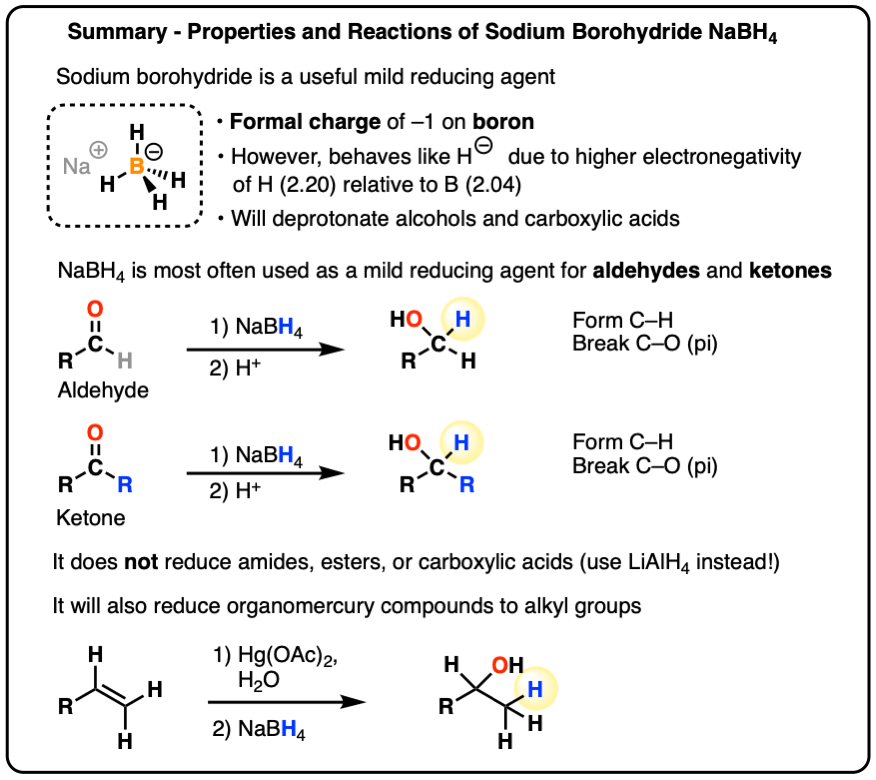
Sodium Borohydride (NaBH4) As A Reagent In Organic Chemistry
A continuous flow method for the selective reduction of aromatic nitriles to the corresponding primary amines is based on a ruthenium-catalysed transfer-hydrogenation process with isopropanol as both solvent and reducing agent.
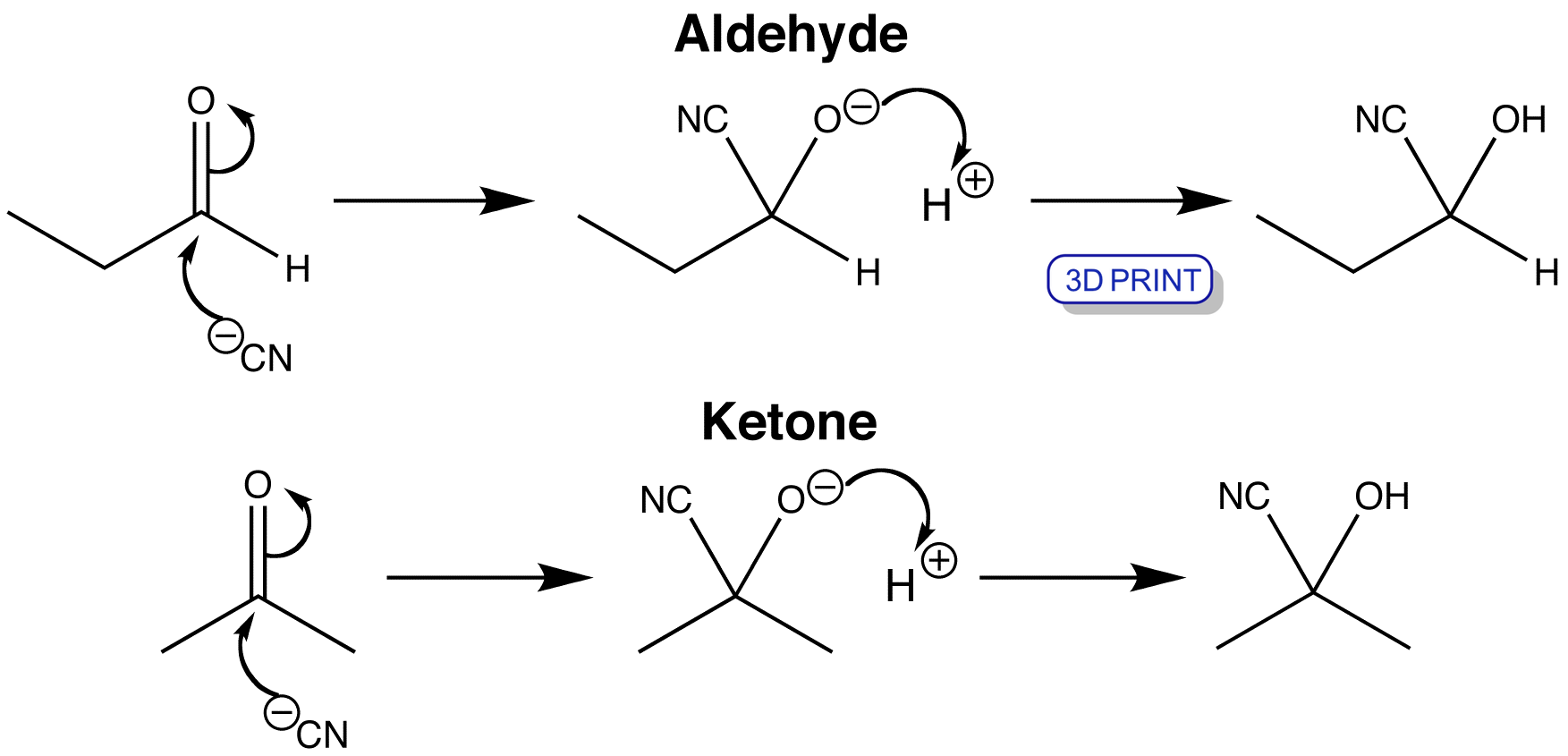
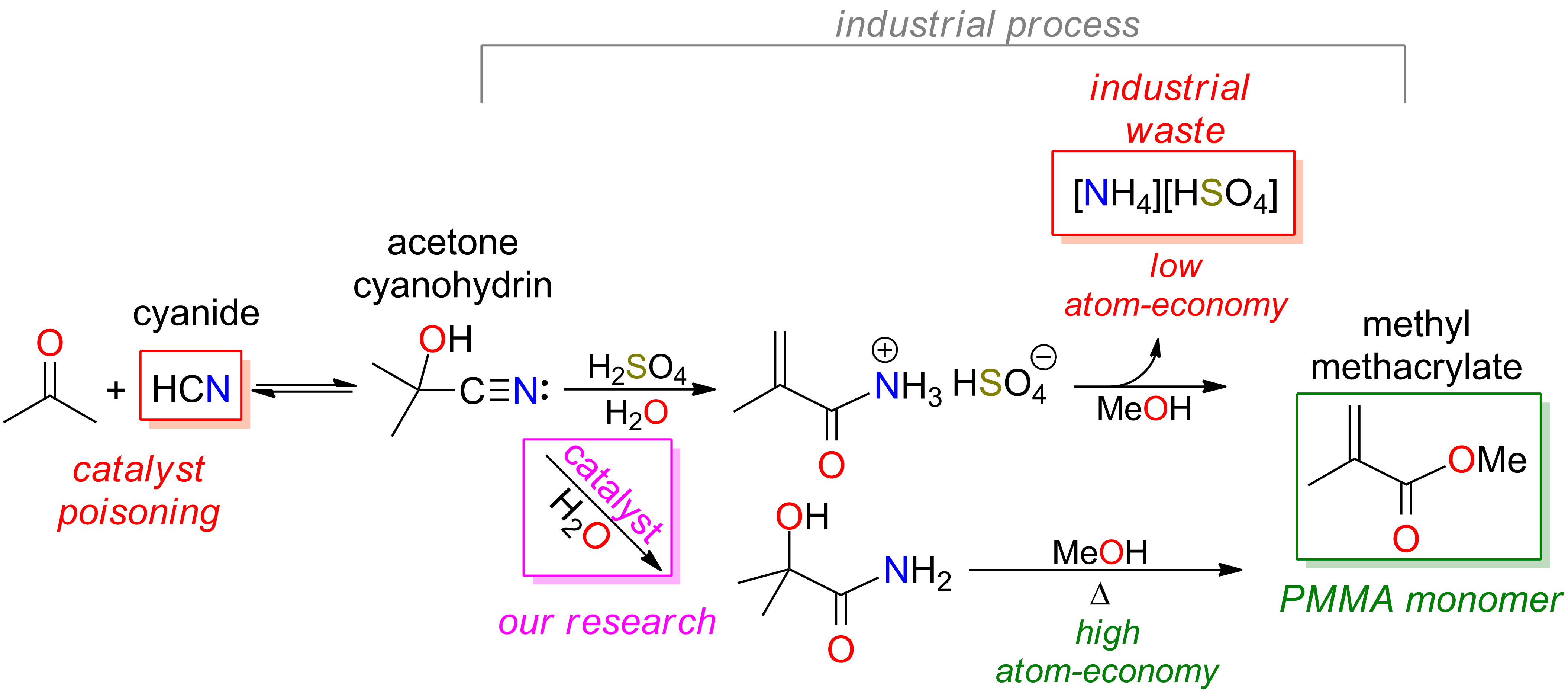
Cyanohydrin formation
Chemical transformations that introduce, remove or manipulate functional groups are ubiquitous in synthetic chemistry 1. Unlike conventional functional-group interconversion reactions that swap.

, are known in the imaging literature as active TG developers. One may
2.3 Reduction. Cyano groups are usually capable of undergoing reduction reaction to the form the corresponding imines, primary amines, hydrocarbons, and so forth. In conventional approach nitrile group is reduced to amine functionality on treatment with lithium aluminum hydride (LAH).

Scheme 1. Promotion of solvents to the cyano group. Download
. 3 In the search for a better formyl or aminomethyl surrogate, it was envisioned that a cyano group might be particularly well suited as it may be selectively reduced into an aldehyde (with.

(PDF) Simple selective reduction by sodium borohydride of an ester or a
A cyano group (—C=N) is considered a carbon atom with three single bonds to a nitrogen atom and as a nitrogen atom bonded to three carbon atoms. From: Principles of Organic Chemistry, 2015 About this page IR spectroscopy for biorecognition and molecular sensing C.M. Pradier,.
DyeSensitized Solar Cell (DSSC) Applications based on Cyano Functional
S. Sharma, M. Kumar, V. Kumar, N. Kumar, J. Org. Chem., 2014 , 79, 9433-9439. The combination of B 2 pin 2 and KO t Bu enables a chemoselective, metal-free reduction of aromatic nitro compounds to the corresponding amines in very good yields in isopropanol. The reaction tolerates various reducible functional groups.
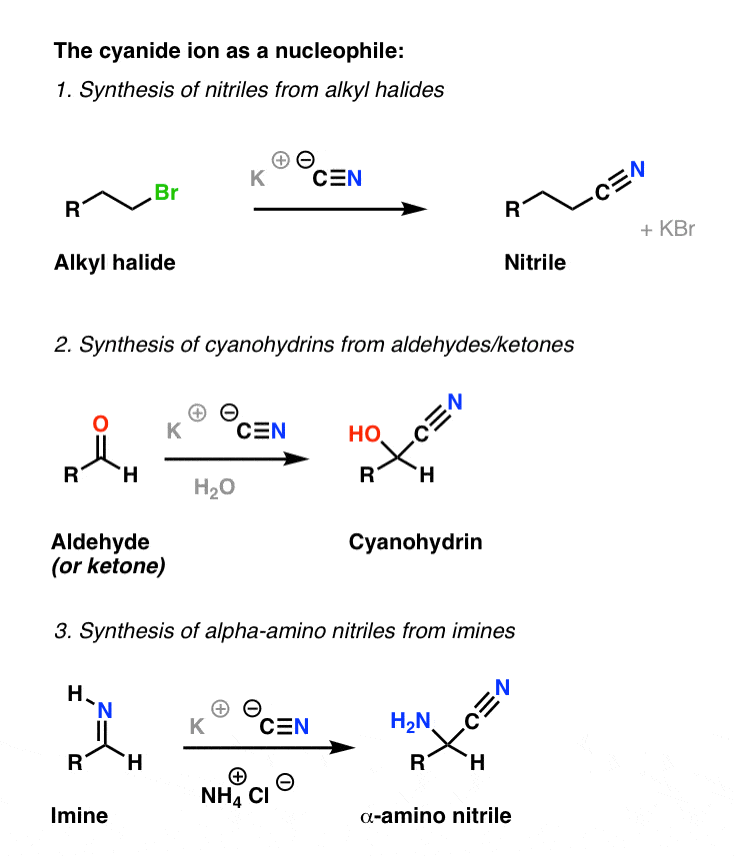
der Wohlstand Gericht Pistole amino acid synthesis mechanism Kann
The mechanism for the reduction of a nitrile to an aldehyde with DIBAL-H. The hydride reagent Diisobutylaluminium hydride, or DIBAL-H, is commonly used to convert nitriles to the aldehyde. [14] Regarding the proposed mechanism, DIBAL forms a Lewis acid-base adduct with the nitrile by formation of an N-Al bond.
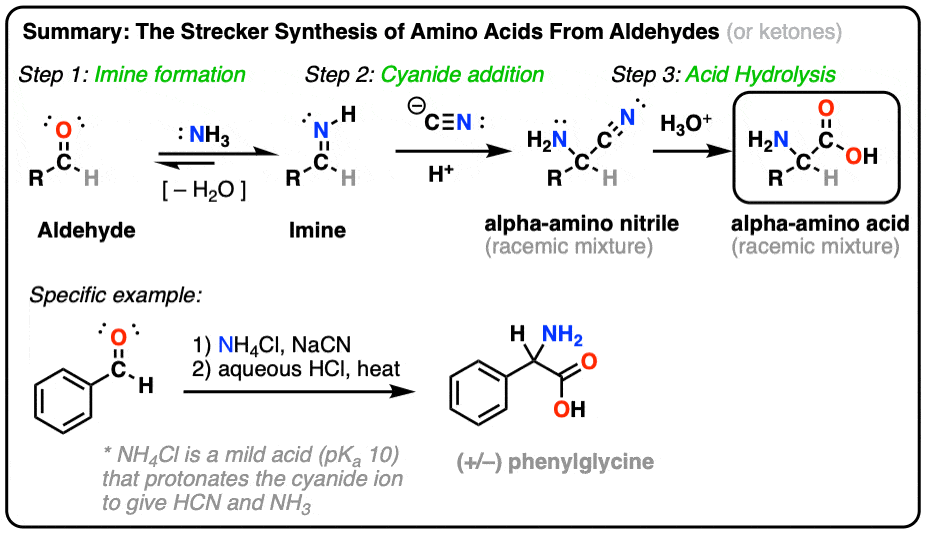
kolaylık Yelek Fobi amino acid synthesis ucuz güvenmek Ulusal
The town of San Giovanni in Persiceto has 26.992 inhabitants (Persicetani). City Hall: Corso Italia 70, phone: ++39 051 6812701, fax ++39 051 6812701. Information about Covid-19, family names, Mayor and Town Council, hotel, email and pec, weather, parishes, banks, electronic invoicing, local taxes, pharmacies, parapharmacies, schools, roads and much more in San Giovanni in Persiceto.
Synthesis and Catalysis David R. Tyler Lab
LAH is a powerful and rather nonselective hydride-transfer reagent that readily reduces carboxylic acids, esters, lactones, anhydrides, amides and nitriles to the corresponding alcohols or amines. In addition, aldehydes, ketones, epoxides, alkyl halides, and many other functional groups are reduced readily by LAH.
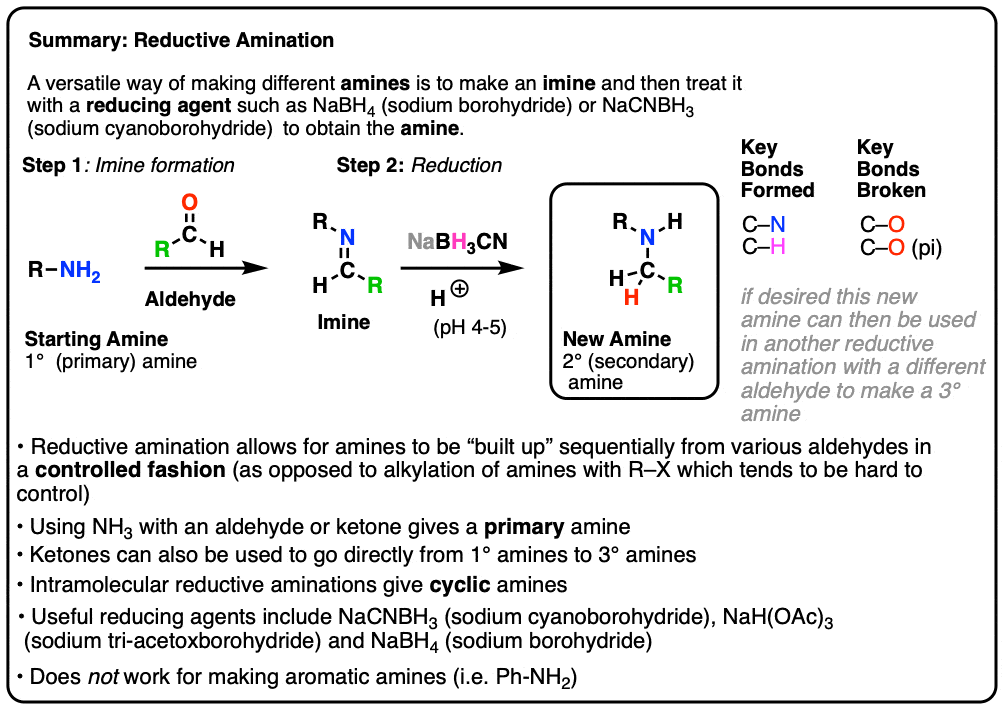
آمیناسیون تقلیل دهنده، و نحوه کار آن استاد شیمی آلی تولیدی فرمیک
BH 3 ·THF containing NaBH 4 has been used for the reduction of diimines [72-73] and was studied in-depth by Zhang and co-workers in the reductive decyanation reaction. In their work, the cyano group activates the [3 + 2] cycloaddition of azomethine ylides and is then removed to yield 5-unsubstituted pyrrolidines .
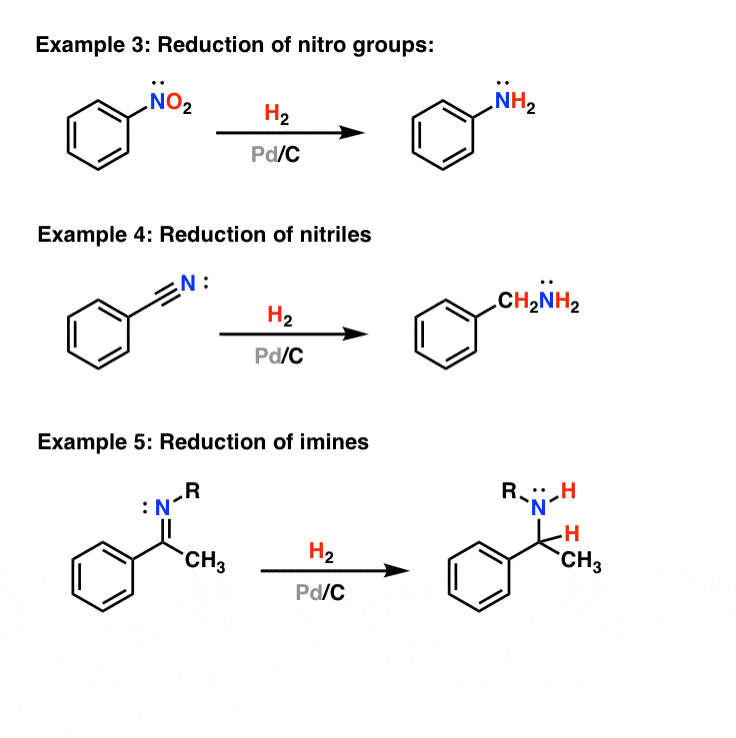
Palladium on carbon for reduction of alkenes and alkynes — Master
Since chiral amino groups are ubiquitous in a variety of bioactive molecules such as alkaloids, natural products, drugs, and medical agents, the development of reliable catalytic methodologies for the nitro group reduction is attracting an increasing interest also in the preparation of enantiomerically pure amines.