
Write the Lewis dot structure of CO molecule.
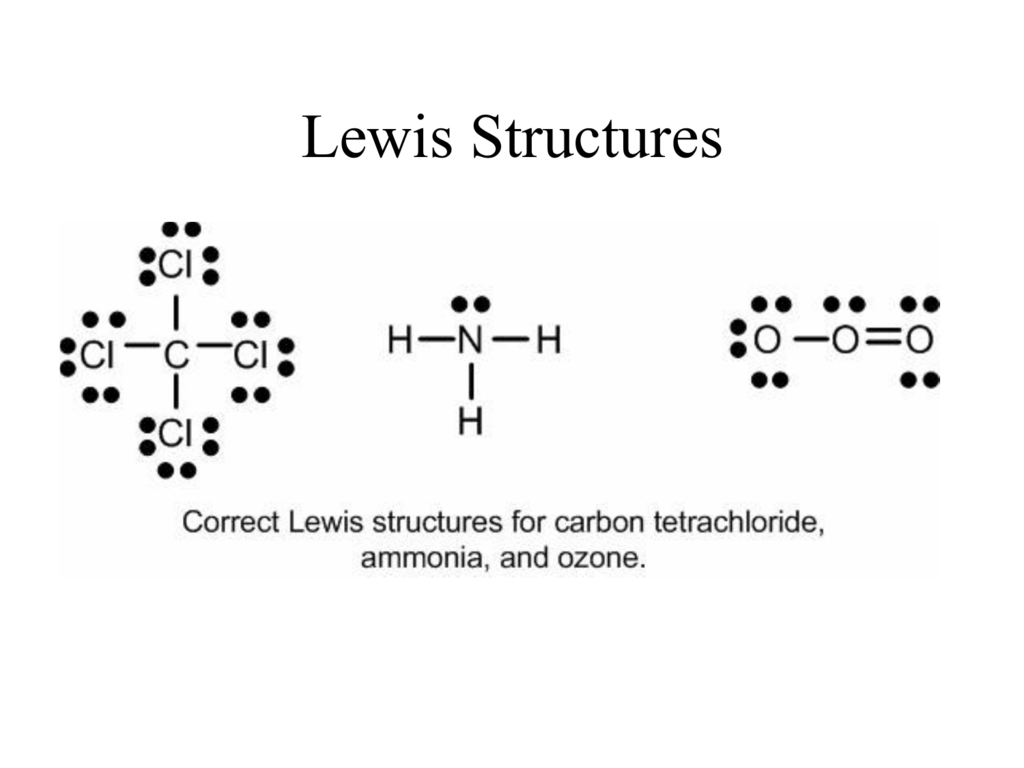
The N atom has the following Lewis electron dot diagram: It has three unpaired electrons, each of which can make a covalent bond by sharing electrons with an H atom. The electron dot diagram of NH 3 is as follows: Exercise 12.4.2 12.4. 2. Use a Lewis electron dot diagram to show the covalent bonding in PCl 3. Answer.
PPT Drawing Lewis Structures A Tutorial on Writing Lewis Dot
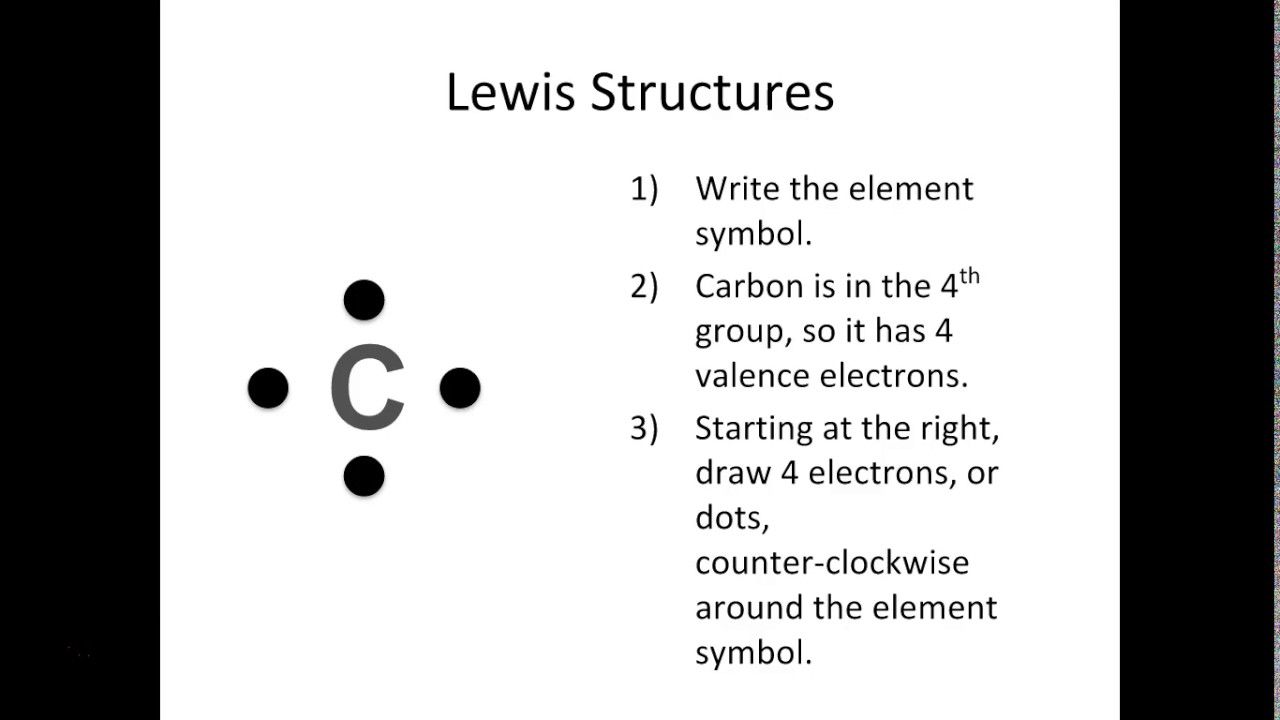
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion.
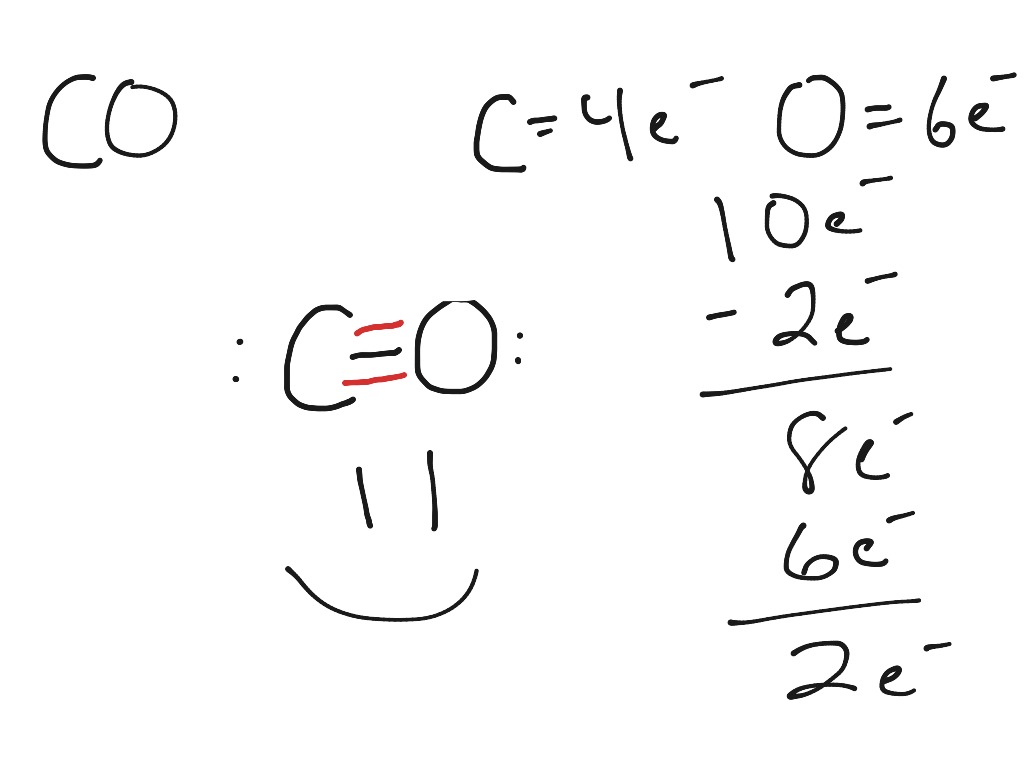
Lewis dot structure of carbon monoxide Science, Chemistry ShowMe
When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share electrons until they are surrounded by eight valence electrons (an octet). Created by Sal Khan. Questions Tips & Thanks Want to join the conversation? Sort by: Top Voted Peter Patterson 3 years ago

Lewis Dot Structure An Overview Borates Today
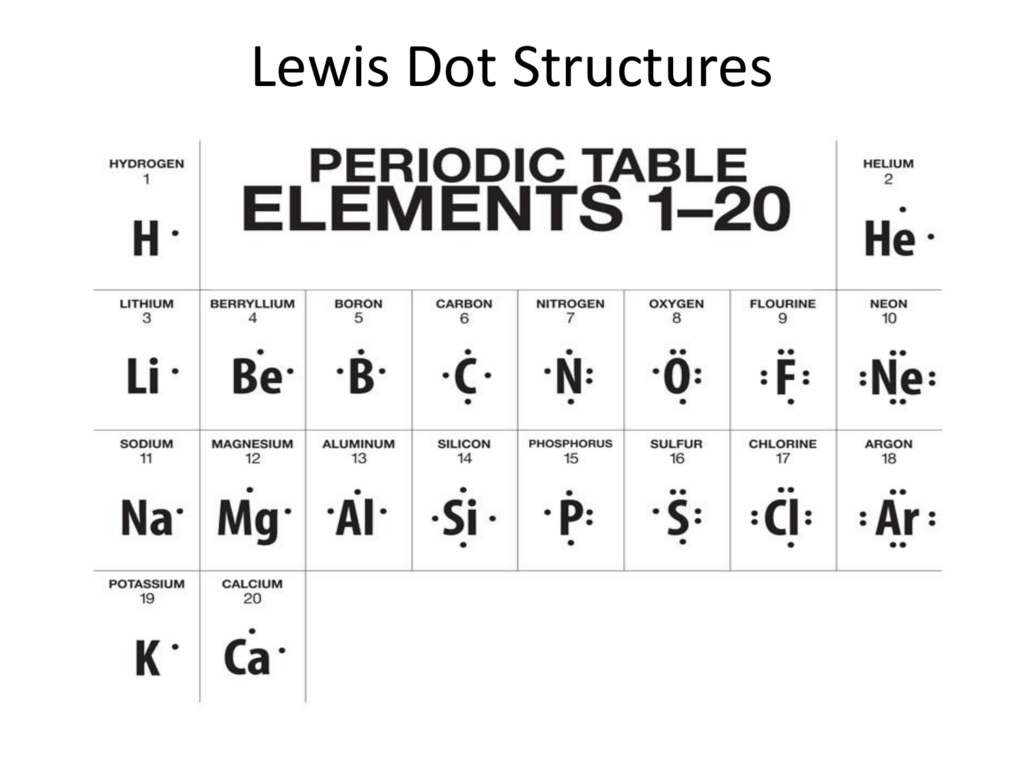
Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures.
Lewis Dot Diagram For N Wiring Diagram
A step-by-step explanation of how to draw the CO Lewis Dot Structure (Carbon monoxide ).For the CO structure use the periodic table to find the total number.
CO Lewis Structure How to Draw the Dot Structure for CO YouTube
Steps of drawing CO lewis structure Step 1: Find the total valence electrons in CO molecule. In order to find the total valence electrons in CO (carbon monoxide) molecule, first of all you should know the valence electrons present in a single carbon atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
14+ Co Lewis Dot Structure Robhosking Diagram
2 days ago Professor Dave Explains Fast-forward to better TV Skip the cable setup & start watching YouTube TV today - for free. Then save $22/month for 3 months. Dismiss Claim offer I quickly.
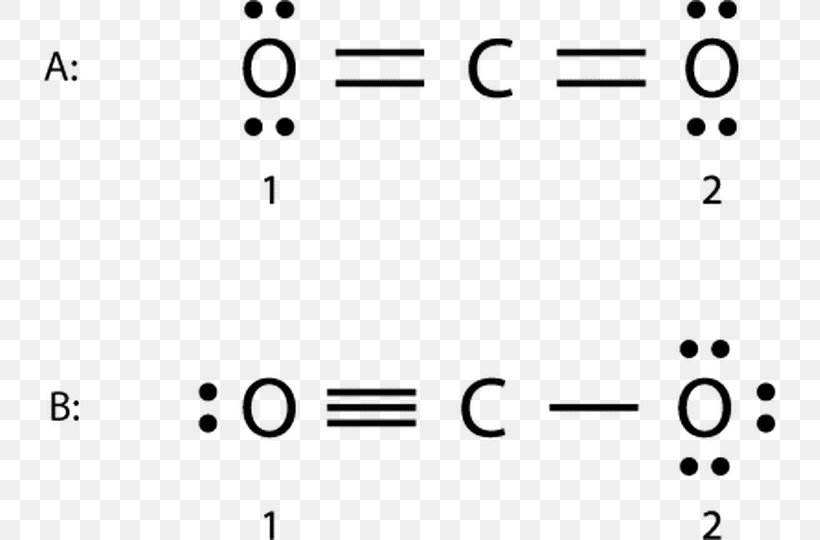
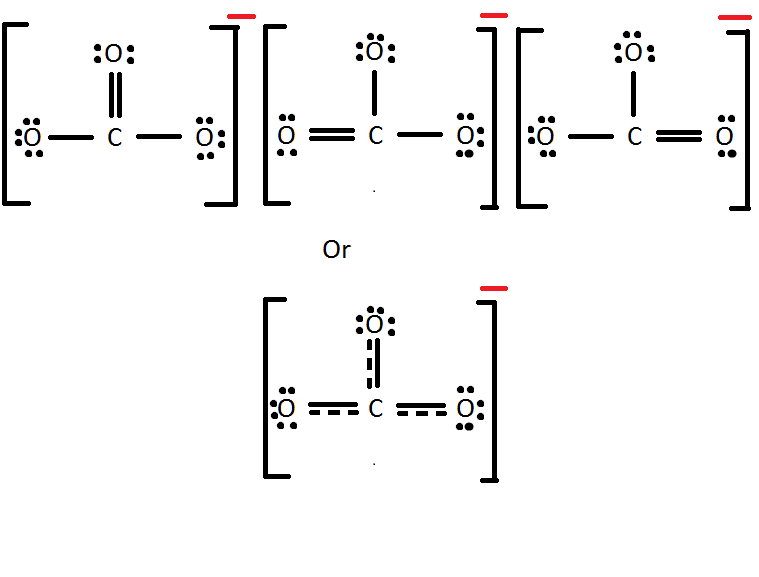
Formal Charge Lewis Structure Resonance Chemistry Carbon Dioxide, PNG
The Lewis structure, also called as electron dot structure, is a simplified method of representing the number of valence electrons present within an atom or a molecule. Furthermore, the structure helps with determining the number of lone pairs of electrons present in an atom and how they act in a bond formation.

Write the Lewis dot structure of CO molecule. Filo
A step-by-step explanation of how to draw the CO Lewis Dot Structure (Carbon dioxide).For the CO structure use the periodic table to find the total number of.
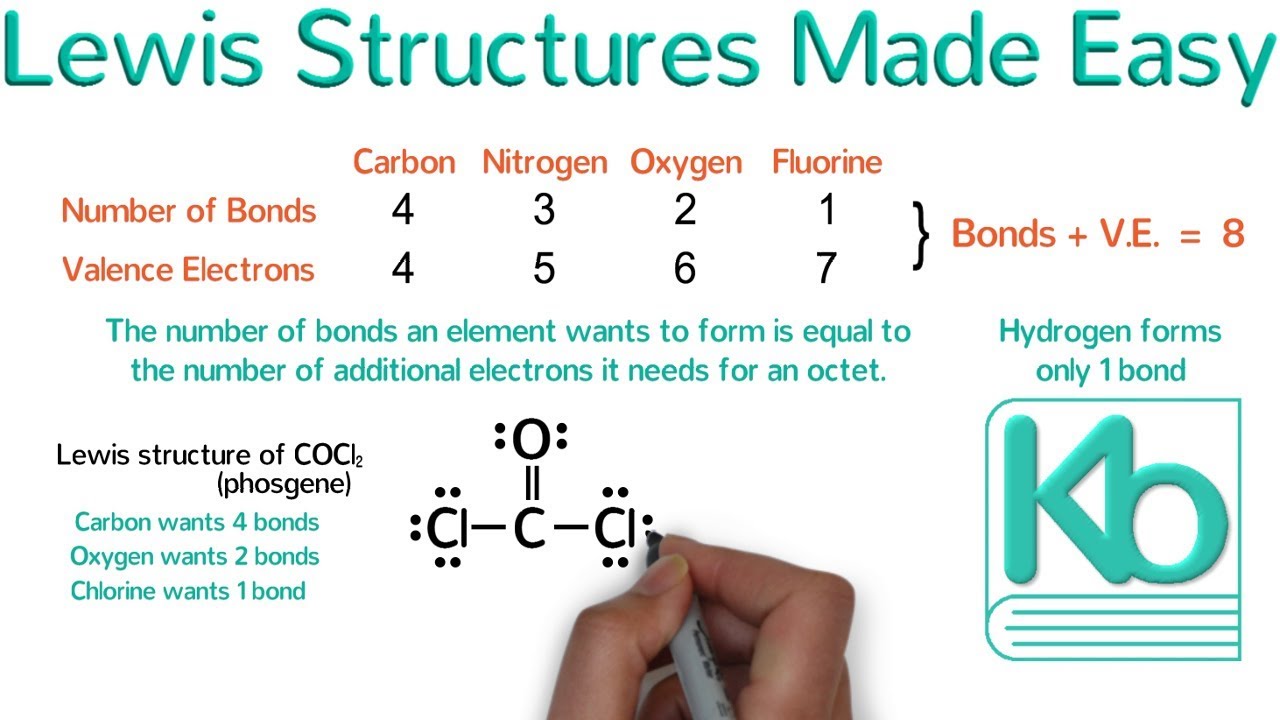
How To Draw Electron Dot Diagrams Elementchampionship Jeffcoocctax
Step-1. Valence electrons Count the total number of valence electrons of carbon and oxygen atom. The outer (valence) shell configuration of carbon and oxygen atoms are: 2s22p2 and 2s22p4 respectively. The valence electrons available are 4+6 =10. Step 2: Skeletal structure The skeletal structure of CO is written as: CO Step 3: Octet completion

Lewis Structure for CO (Carbon Monoxide) Carbon monoxide, Ap
this is the complete Lewis structure of CO 2. For Lewis structure purposes, the lone-pairs can only be moved from terminal atoms to the central atom to form multiple bonds, not the other way around. 7. Formal charges check: all atoms have formal charges equals to 0 in this structure. FC (C) = 4 -½× (4×2) = 0.
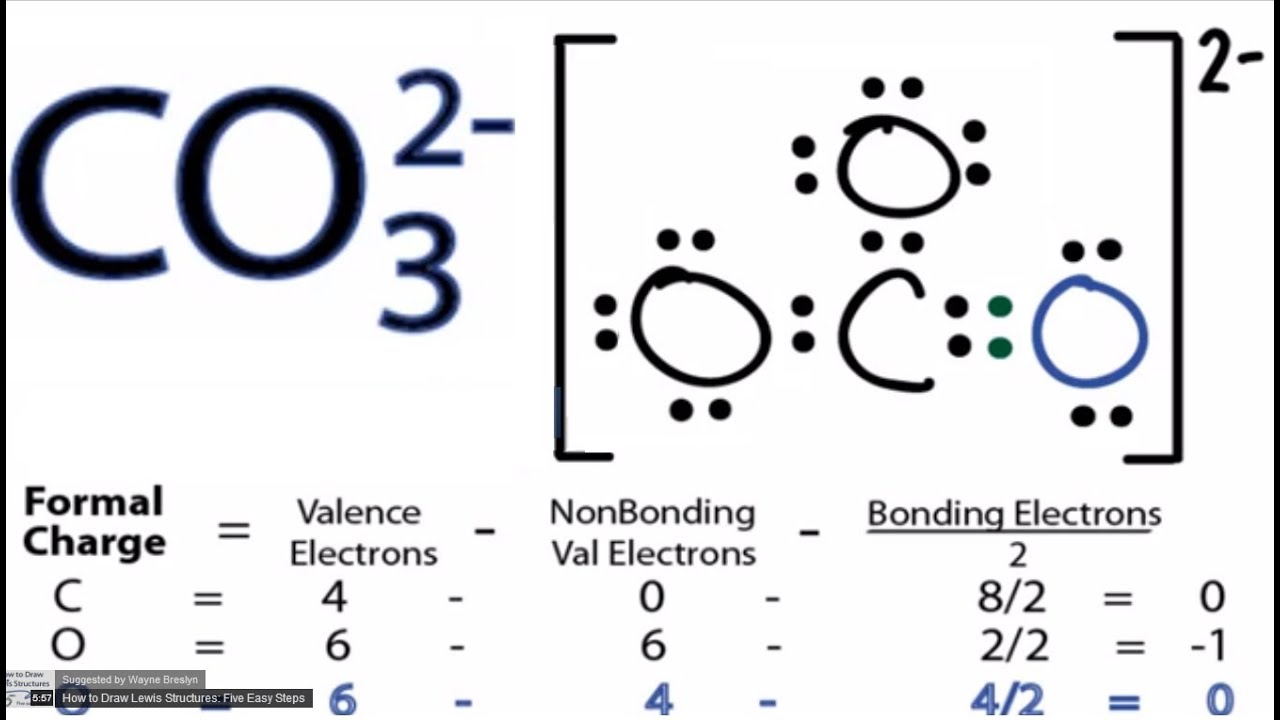
CO32 Lewis Structure How to Draw the Lewis Structure for CO3 2
For the CO Lewis structure there are a total of 10 valence electrons available. Transcript: This is the CO Lewis structure: Carbon monoxide. We have 4 valence electrons for Carbon and 6 for Oxygen, for a total of 10 valence electrons. So we have a Carbon and an Oxygen atom bonded together. We'll put 2 electrons between the atoms to form a.
Lewis dot structures YouTube
1. Count electrons 2. Put least electronegative atom in centre.more.more The Lewis Structure (Lewis Dot Diagram) for CO.1. Count electrons2. Put least electronegative atom in centre3..

Chapter 4 Part 2 Lewis Structures Chemistry 1120 with Bjorkman at
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Lewis Theory of Bonding Chemistry LibreTexts
Carbon monoxide (CO) is a diatomic molecule and contains one carbon atom and one oxygen atom. Lewis structure of CO molecule contains a triple bond between those two atoms. Both Carbon and Oxygen atoms have one lone pair in their valence shells. Lewis structure of Carbon monoxide (CO) molecule
Lewis Dot Structures
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".